Effective Ways to Name Ionic Compounds
In this article, we will delve into discovering modern techniques for clarity in naming **ionic compounds** as of 2025. Understanding the naming conventions is crucial for clarity in chemistry.
Understanding Ionic Compounds and Their Characteristics
Ionic compounds are formed through the electrostatic attraction between **cations** and **anions**. A **cation** is a positively charged ion, such as sodium (Na+), while an **anion** is a negatively charged ion, like chloride (Cl–). When these ions combine, they form a stable ionic bond which produces a **formula unit**. The resulting compounds, such as sodium chloride (NaCl), exhibit distinct **ionic compound properties** like high melting points, solubility in water, and electrical conductivity when dissolved. This stability and predictability emphasize the importance of a systematic approach to **naming ionic compounds**, where precision is paramount for understanding chemical formulas.
The Role of Cations and Anions in Naming
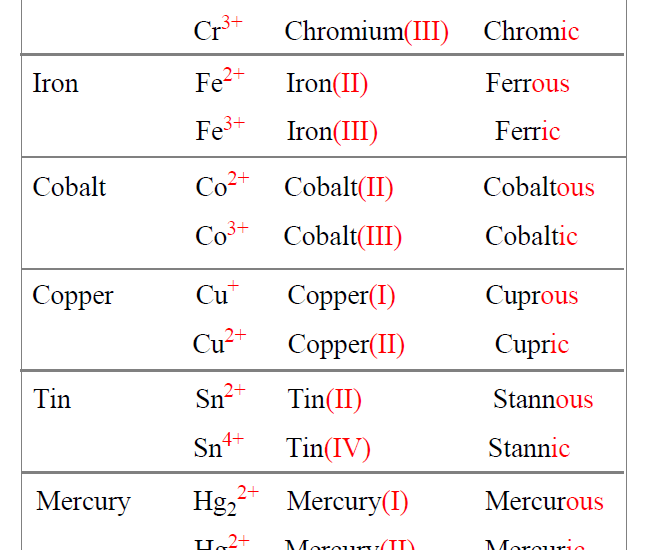
When **naming ionic compounds**, the first step is identifying the cation and anion involved. For instance, in magnesium oxide (MgO), magnesium (Mg2+) acts as the cation while oxide (O2-) is the anion. It’s essential to consider the **oxidation states** of these ions, especially when dealing with **transition metals**. Unlike metals with fixed charges, transition metals can have multiple oxidation states, complicating the naming process. Therefore, we often use **Roman numerals** to indicate the charge, such as in iron(III) oxide (Fe2O3), where iron carries a +3 charge.
Importance of Charge Balance in Ionic Nomenclature
Proper naming of ionic compounds demands attention to **charge balance**. The total positive charge from the **cation** must equal the total negative charge from the **anion**. For example, calcium chloride is derived from calcium (Ca2+) and two chloride ions (2 Cl–), which balances the charges perfectly (CaCl2). This systematic approach also extends to those incorporating **polyatomic ions**, groups of atoms that carry a collective charge, such as sulfate (SO42-). Understanding how to integrate polyatomic ions is vital for correct nomenclature.
Rules for Naming Ionic Compounds
The naming of ionic compounds follows a few established rules that enhance clarity in communication. Familiarity with these rules fosters understanding and retention among students and practitioners alike.
Simple Rules for Binary Ionic Compounds
Binary ionic compounds consist of two types of ions: a **metal** and a **non-metal**. The rule is straightforward: the metal name precedes the anion name, adjusted to a suitable suffix. For non-metals, the conventional practice is to alter the suffix to -ide. Boron nitride (BN) reflects this because boron is the metal and nitrogen forms the corresponding anion. It’s simple yet effective, ensuring common compounds are easily identifiable.
Nomenclature of Compounds with Transition Metals
When dealing with transition metals, the rules for naming vary slightly. These metals can exhibit different oxidation states, thus necessitating Roman numerals to denote the cation’s charge specifically. For example, in copper(II) sulfate (CuSO4), the Roman numeral II signifies that copper has a +2 oxidation state. Teaching and practicing this approach to **naming ionic compounds** involving transition metals can aid in player collaboration in a laboratory environment, making it easier to follow chemical reactions and protocols.
Incorporating Polyatomic Ions Into Nomenclature
Beyond binary ionic compounds, **polyatomic ions** should also be understood and embraced in modern nomenclature. Polyatomic ions consist of multiple atoms that together share a charge. Familiar examples include ammonium (NH4+) and hydroxide (OH–). Understanding how these fit into compound naming, such as in ammonium chloride (NH4Cl), broadens comprehension and establishes a solid foundation for future **chemistry** learning. Utilizing specific resources may enhance the teacher’s toolkit to fortify this understanding.
Practical Examples and Tips for Naming Ionic Compounds
To reinforce understanding, practical examples and tips for naming ionic compounds are invaluable resources for both educators and learners in chemistry.
Naming Salts and Common Ionic Compounds
The processes involved in naming ionic compounds often extend into real-world applications such as the formulation of **salts**. **Salts** are ionic compounds formed from the neutralization reaction of acids and bases, and their nomenclature follows established rules. Examples include sodium phosphate (Na3PO4) and potassium chloride (KCl). Familiarizing ourselves with these compounds, along with using flashcards or interactive tools, can greatly assist students in mastering the **understanding of ionic compounds**.
Teaching Techniques to Enhance Learning
Incorporating visual aids such as diagrams or structured charts can significantly improve the teaching of **naming ionic compounds**. For example, utilizing animation that illustrates the process of electron transfer and ionic bond formation can capably demonstrate theoretical concepts. Additionally, problem-solving exercises could assist students in applying naming rules while reinforcing their understanding. Online platforms and educational resources containing practice quizzes test their knowledge of nomenclature. Utilizing Nomenclature helpbooks may also guide students in their studies effectively.
Wild Cards and Exceptions in Naming Ionic Compounds
As with any rule set, there will always be exceptions or rules interspersed with terms that may demonstrate unique characteristics. For instance, the nomenclature of hydrates – ionic compounds that include water molecules in their crystal structure (e.g., copper(II) sulfate pentahydrate, CuSO4•5H2O) – forms a necessary comprehension layer in modern ionic compound education. Exposing students to these exceptions encourages overall adaptability in their learning processes, which expands their views beyond strict rules.
Key Takeaways
- Understanding ionic compounds involves knowing the characteristics of cations and anions, as well as their charges.
- The rules for binary ionic compounds, including the use of prefixes and suffixes, are foundational for naming conventions.
- Proper nomenclature for transition metals and polyatomic ions enhances clarity and communication in chemical descriptions.
- Practical examples like naming salts and teaching methods can improve student engagement and comprehension of the subject.
FAQ
1. What are the common ionic compounds found in daily life?
Common ionic compounds include table salt (sodium chloride, NaCl), baking soda (sodium bicarbonate, NaHCO3), and calcium carbonate (CaCO3), which can be easily identified even in household settings. Many of these ionic compounds play vital roles in chemical reactions essential for everyday processes.
2. How do oxidation states affect the naming of ionic compounds?
Oxidation states determine the charge of the ions in an ionic compound, which is critical for naming. Knowing if a metal ion can have multiple oxidation states affects the use of Roman numerals in naming, thus signifying the ion’s specific charge in its molecular form.
3. Can ionic compounds be found in biological systems?
Yes, ionic compounds such as sodium chloride and potassium iodide play significant roles in biological systems, being pivotal for neural function and cellular metabolism. Understanding these compounds opens a door to their relevance in health and medicine.
4. What are the properties of ionic compounds?
Ionic compounds typically exhibit high melting and boiling points due to the strong electrostatic forces between the ions. Additionally, they are usually soluble in water and can conduct electricity when dissolved, distinguishing them from covalent compounds.
5. Why is understanding ionic compound nomenclature important?
Mastering the nomenclature of ionic compounds reinforces foundational knowledge in chemistry, enabling accurate communication, understanding of chemical formulas, and the ability to predict and describe chemical behaviors in reactions. It also aids in further studies of advanced chemical concepts.